The first comprehensive ERP software for pharmaceutical manufacturing in the world, Paperless GMP is combined with a feature-rich Quality Management System (QMS). This verified ERP offers offline and cloud-based solutions designed to meet the requirements of the pharmaceutical sector while adhering to ALCOA data integrity rules and 21 CFR Part 11.
Our Paperless Training Software, a cutting-edge technology to digitize and automate GMP, SOP, and compliance training in the pharmaceutical business, will help you streamline your workforce training. Completely compliant with 21 CFR Part 11, WHO-GMP, and EU-GMP, this solution guarantees digital progress tracking, audit readiness, consolidated personnel records, and trustworthy training documentation.
Available in Cloud Mode
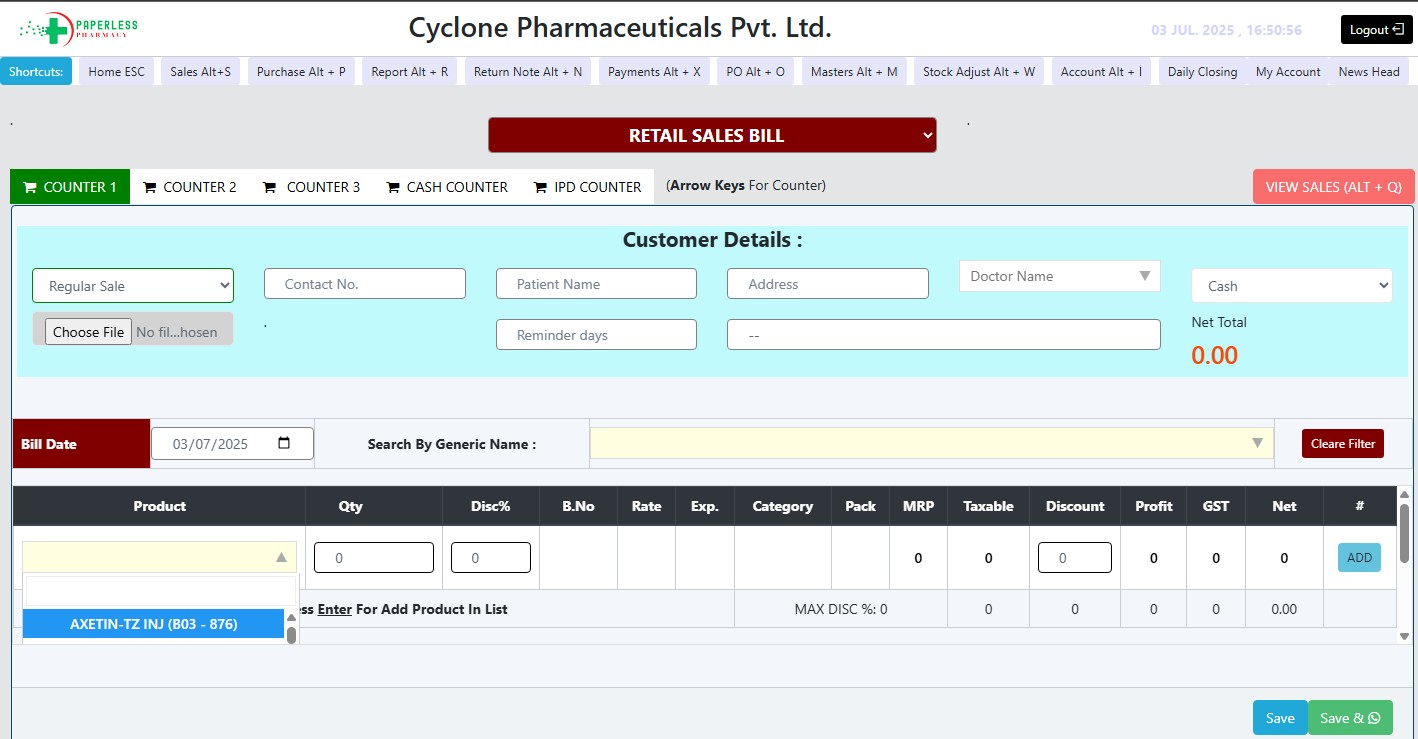
Useful for More Than One Counter
Smart Prescription Management
Bills on Whatsapp
Patient Reminder
One click Purchase Bill Import
All FDA Reports Available
Return Bill
Short Bill & Customized PO
Opening Stock & Stock Adjustment
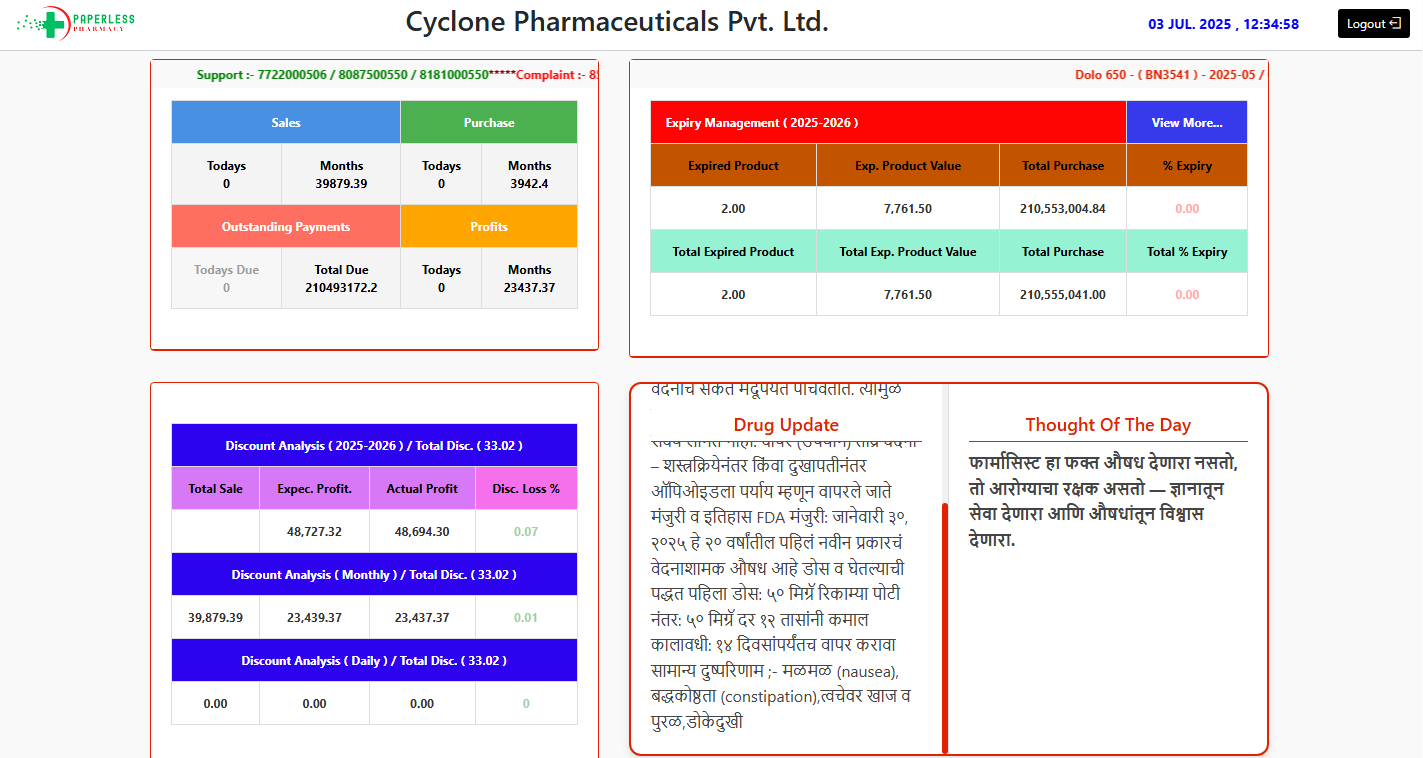
Manage Complate Account of Pharmacy
Daily Closing Report
QR Code Billing
Expense Tracking & Profit Analysis
Established in 2016, GMP Software India is a sister concern of Cyclone Pharmaceuticals Pvt. Ltd., a leader in pharmaceutical technology and GMP compliance
Leveraging over 14 years of industry expertise, we focus on advancing digital pharmaceutical documentation and streamlining Quality Management Systems (QMS).
As Cyclone Pharmaceuticals Pvt Ltd India's software subsidiary, GMP Software India specialises solely in creating software solutions for the pharmaceutical sector. The comprehensive solution for pharmaceutical manufacturing, marketing, lab management, QMS, and administrative tasks is one of their hallmark products.